Biologics Exclusivity: What It Means for Drug Prices and Treatment Access
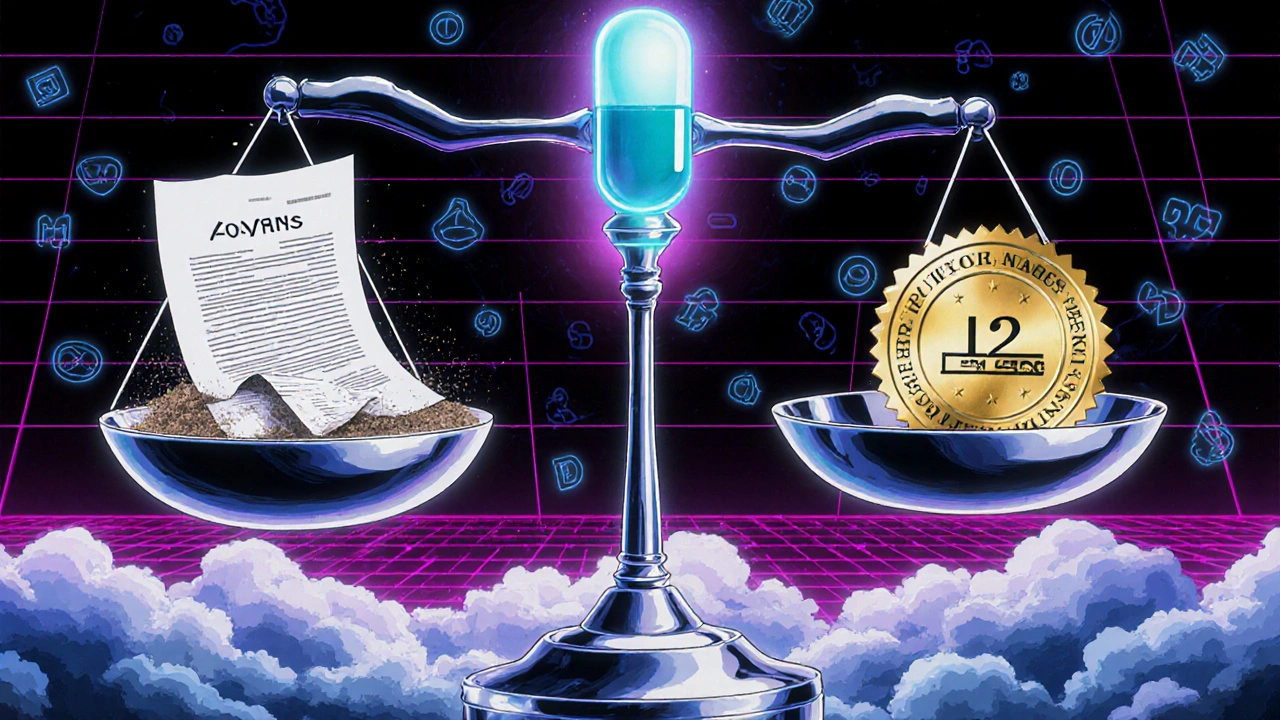
When you hear biologics exclusivity, a period of market protection granted to brand-name biologic drugs to recoup research costs before generics can enter. Also known as data exclusivity, it’s the legal shield that keeps cheaper versions off shelves for years—even after the patent expires. Unlike regular pills, biologics are made from living cells, so copying them isn’t as simple as swapping ingredients. That’s why regulators treat them differently—and why this exclusivity period matters so much to your wallet and your health.
Biologics exclusivity directly impacts biosimilars, lower-cost versions of biologic drugs that are highly similar but not identical to the original. These aren’t generics—they’re more like close relatives. In the U.S., the exclusivity window is 12 years, meaning no biosimilar can launch until after that time. In Europe, it’s 8 years plus 2 more for market protection. That delay means patients pay thousands more per year for drugs like Humira or Enbrel, even when science says a cheaper version could work just as well. Meanwhile, biologic therapies, treatments made from proteins or antibodies that target specific parts of the immune system. Also known as targeted biologics, they’re used for rheumatoid arthritis, Crohn’s disease, psoriasis, and even some cancers. These drugs changed how we treat chronic conditions—but their high prices have sparked debates about fairness, access, and whether exclusivity is helping innovation or just protecting profits.
The posts below dig into real-world examples of how this plays out. You’ll find comparisons between biologics and their alternatives—like methotrexate versus biologics for rheumatoid arthritis, or how biosimilars stack up against brand-name drugs in cost and effectiveness. Some posts look at how these rules affect treatment choices, while others break down why certain drugs stay expensive even when the science is old. Whether you’re managing a chronic illness, paying out of pocket, or just trying to understand why your prescription costs so much, this collection gives you the facts without the jargon.