MedWatch: Your Guide to Drug Safety and Regulatory Alerts
When you take a medication, you trust it’s safe—but what if something dangerous wasn’t caught until after thousands of people used it? That’s where MedWatch, the FDA’s safety monitoring program that collects reports of adverse drug reactions and product problems. Also known as FDA MedWatch, it’s the system doctors and pharmacists rely on to spot hidden risks in medicines after they hit the market. This isn’t just paperwork—it’s the last line of defense when clinical trials miss real-world side effects, like liver damage from a new statin or heart rhythm problems from an anti-nausea drug.
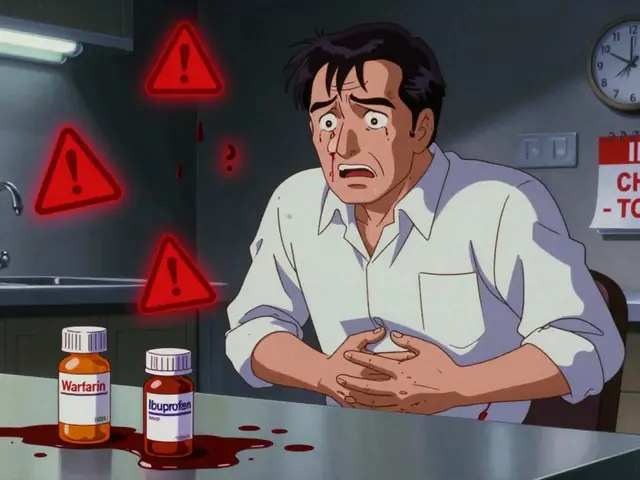
MedWatch doesn’t work alone. It’s tied to other critical systems. For example, regulatory exclusivity, the legal barriers that let drugmakers block generics even after patents expire. This delays cheaper options and keeps patients paying more, even when the original drug has known risks like QT prolongation or liver toxicity. Then there’s counterfeit drugs, fake or substandard medications that slip into the supply chain because sourcing standards aren’t enforced. These aren’t rare—they show up in online pharmacies and even some local stores, especially for high-demand drugs like insulin or blood pressure pills. And when these fake pills mix with real ones, the result? Dangerous interactions, like Ginkgo biloba thinning your blood too much when paired with warfarin, or diuretics spiking lithium levels to toxic levels.
MedWatch also connects to everyday safety practices. School nurses use the Five Rights to avoid giving kids the wrong dose. Older adults check the Beers Criteria before taking generics. People on opioids get naloxone co-prescribed. All of these are human-scale responses to system-level risks. When a drug causes neuroleptic malignant syndrome or triggers serotonin syndrome with OTC cold meds, those reports go into MedWatch. And when insurers push bulk-buying generics that vary in fillers and dyes, causing intolerances in sensitive patients, those stories matter too. MedWatch turns individual experiences into national alerts.
You don’t need to be a doctor to use MedWatch. If you’ve had a strange reaction to a pill—rash, dizziness, swelling, or worse—you can report it. You can ask your pharmacy about sourcing standards or demand easy-open caps if you’re struggling with child-resistant lids. You can check if your medication is affected by regulatory exclusivity that keeps prices high. The system only works if people speak up. Below, you’ll find real stories from patients and providers about the hidden dangers in common meds, how to spot bad interactions, and what to ask before you take anything new. These aren’t theory—they’re lessons learned from real cases tracked by MedWatch.