Immunosuppressant Monitoring Calculator
How This Drug Is Monitored
When you're on immunosuppressive drugs like tacrolimus, cyclosporine, or mycophenolate, you're not just taking medication-you're managing a tightrope walk between preventing rejection or flare-ups and avoiding dangerous side effects. These drugs don't have a one-size-fits-all dose. Two people taking the same pill, at the same time, can have wildly different blood levels. One might be perfectly protected from rejection, while the other is at risk of kidney damage or a serious infection. That’s why monitoring isn’t optional-it’s life-saving.
Why Routine Monitoring Matters
Immunosuppressants have a razor-thin window between working and harming. For tacrolimus, the difference between a safe level and a toxic one is often less than 2 ng/mL. Too low? Your body might attack the transplanted organ. Too high? You risk kidney failure, nerve damage, or even diabetes. The same goes for cyclosporine and sirolimus. Without regular checks, you're flying blind. A 2022 study from the American Society of Transplantation showed that patients on monitored therapy had 37% fewer acute rejections and 22% better five-year organ survival than those on fixed doses. That’s not a small improvement-it’s the difference between living with a functioning transplant and needing another one.Therapeutic Drug Monitoring: The Core of Safe Treatment
Not all immunosuppressants need the same kind of tracking. Calcineurin inhibitors like tacrolimus and cyclosporine, plus mTOR inhibitors like sirolimus and mycophenolic acid (MPA), require regular blood tests to measure drug levels. Steroids like prednisone and newer drugs like belatacept don’t need this level of monitoring because their effects are less dependent on precise blood concentrations. For tacrolimus, the standard is checking the trough level-the lowest concentration in your blood, just before your next dose. In the first three months after a kidney transplant, doctors aim for 5-10 ng/mL. After that, they lower it to 3-7 ng/mL to reduce long-term kidney damage. Cyclosporine is trickier. Some centers still check just the trough, but the best predictor of rejection is the C2 level-the concentration two hours after taking the dose. Studies show C2 monitoring correlates much better with outcomes than trough levels alone. Mycophenolic acid (MPA) is another challenge. Its levels fluctuate because of how your gut and liver process it. Just checking the trough isn’t enough. The real measure is the area under the curve (AUC), which tracks how much drug is in your system over time. An AUC between 30 and 60 mg·h/L is linked to an 85% chance of staying rejection-free in the first year. Testing methods matter too. Immunoassays are cheaper and faster, but they can overestimate levels by 15-20% because they react with drug byproducts. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the gold standard. It’s more accurate, more precise, and doesn’t get fooled by metabolites. The downside? It costs $150-$250 per test, compared to $50-$100 for immunoassays. Many centers still use the cheaper option because of budget limits, even though it risks mismanagement.Lab Tests Beyond Drug Levels
Monitoring isn’t just about the drug in your blood. It’s about what the drug is doing to your body. Every 1-3 months, you’ll need a full panel of blood tests:- Full blood count: To catch low white blood cells, anemia, or low platelets-common with mycophenolate and sirolimus.
- Creatinine and electrolytes: To track kidney function. Cyclosporine and tacrolimus can cause up to a 30% rise in creatinine in 25% of patients.
- Liver enzymes: To spot early signs of liver stress.
- Calcium, magnesium, phosphate: Cyclosporine causes magnesium loss in 40-60% of patients. Low magnesium can lead to muscle cramps, irregular heartbeat, or seizures.
- Fasting glucose: Tacrolimus increases diabetes risk by 30% compared to cyclosporine. Monitoring fasting sugar helps catch this early.
- Lipids: Sirolimus causes high cholesterol and triglycerides in 60-75% of users. Annual lipid panels are critical to prevent heart disease.
- Mycophenolate: Watch for diarrhea (30-40% of patients) and low white blood cells (25-30%).
- Sirolimus: Watch for lung inflammation (pneumonitis in 1-5%), low platelets, and high cholesterol.
- Cyclosporine: Watch for tremors, headaches, and gum swelling-signs of neurotoxicity or overexposure.
Imaging: Seeing What Blood Tests Can’t
Some problems don’t show up in a blood test. That’s where imaging comes in.- Renal ultrasound: Done at least once a year-or anytime your creatinine rises. It checks for blockages, scarring, or reduced blood flow to the transplanted kidney.
- Chest X-ray: If you develop a persistent cough or shortness of breath, this is the first step to rule out pneumonitis, a known side effect of sirolimus and everolimus. It’s not perfect-only 70-85% sensitive-but it’s fast and widely available.
- Bone density scan (DEXA): Corticosteroids weaken bones. After one year of steroid use, annual scans are recommended to catch osteoporosis before you break a hip or vertebra.
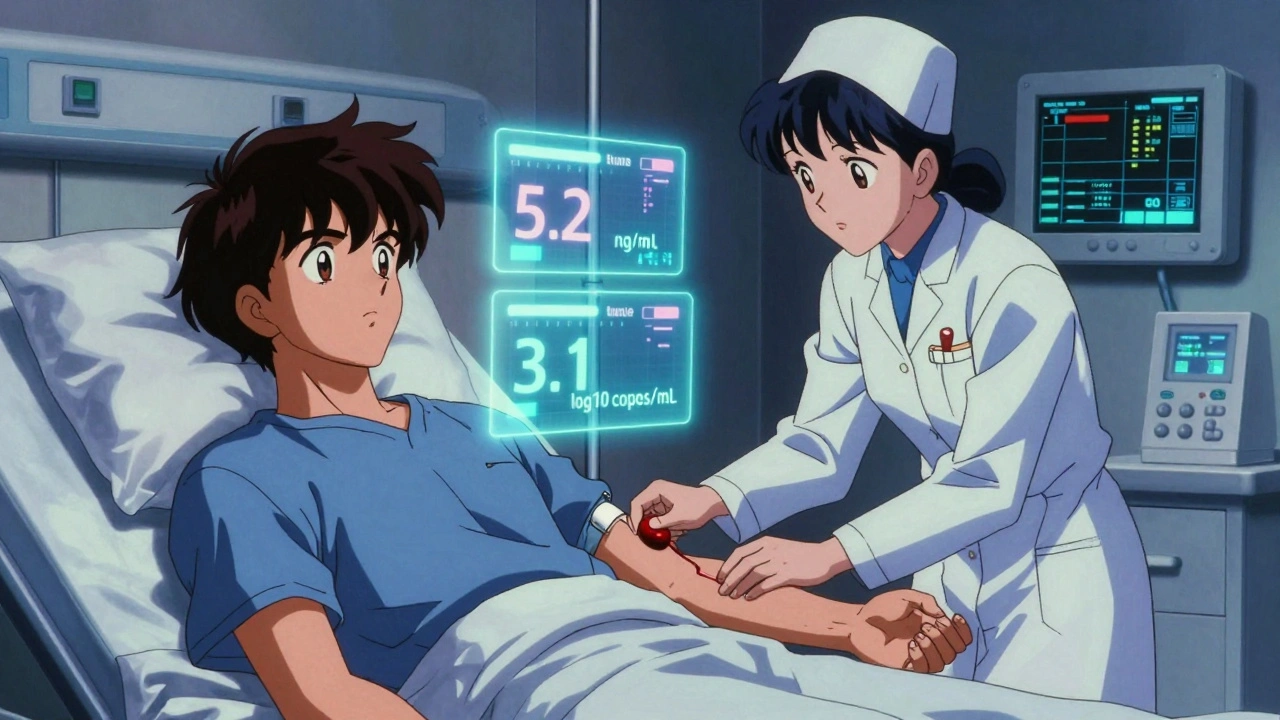
The Future Is Here: TTV Monitoring
The most exciting development in immunosuppression monitoring isn’t a new drug-it’s a virus you didn’t know you had. Torque Teno Virus (TTV) is harmless to healthy people. But in transplant patients, it multiplies freely because your immune system is suppressed. The more TTV in your blood, the weaker your immune response. Researchers found a clear pattern: a TTV load between 2.5 and 3.5 log10 copies/mL is the sweet spot. Below that? You’re at higher risk of rejection. Above it? You’re more likely to get a serious infection. A major trial called TTVguideIT, running across multiple countries, found that adjusting drug doses based on TTV levels led to 28% fewer infections and 22% fewer rejections compared to standard care. It’s not perfect yet-labs don’t all use the same test-but it’s the closest thing we have to a real-time immune system gauge.Challenges in Real-World Practice
Even with all this science, many centers struggle to do it right. A 2022 survey of 150 transplant centers found that 68% had no consistent monitoring rules across departments. Only 42% had standardized protocols for MPA monitoring. Why?- Cost: LC-MS/MS tests are expensive. Many hospitals can’t afford them for everyone.
- Lack of standardization: There’s no universal target for sirolimus levels. Some centers use 5-10 μg/L. Others use 7-12. No one agrees.
- Staff training: Nurses and pharmacists need to understand how to interpret these complex results. Many don’t get enough training.

What Works Best: A Team Approach
The best outcomes happen when pharmacists, nurses, and doctors work together. Centers with dedicated immunosuppression teams-people who review every lab result within 24 hours-see the fewest complications. These teams adjust doses before problems arise, not after. Artificial intelligence is starting to help too. A 2023 study used machine learning to analyze trends in tacrolimus levels, TTV, and routine labs. The algorithm predicted rejection 14 days before symptoms showed up-with 87% accuracy. That’s not science fiction anymore. It’s happening in hospitals right now.What’s Next?
The future is moving toward personalized, predictive monitoring. Point-of-care devices that give you drug levels from a finger-prick blood drop are in trials and could be available by 2027. Non-invasive methods, like analyzing your breath for drug metabolites, are being tested in labs. And regulatory agencies are preparing to approve TTV testing as a clinical tool-possibly as early as 2025. The bottom line? Monitoring isn’t just checking boxes. It’s the foundation of long-term survival after transplant or for managing autoimmune disease. It’s not about how much drug you take-it’s about how your body responds to it. And that changes every day.How often do I need blood tests for immunosuppressants?
In the first 3 months after transplant, you’ll likely have blood tests every 1-2 weeks to fine-tune your dose. After that, it usually drops to once every 2-4 weeks for the next 6-12 months. Once you’re stable (usually after 1 year), most people need tests every 1-3 months. If your doctor changes your medication or you get sick, you may need more frequent testing.
Can I skip my lab tests if I feel fine?
No. Many side effects of immunosuppressants-like rising creatinine, low magnesium, or early signs of diabetes-don’t cause symptoms until they’re advanced. Feeling fine doesn’t mean your drug levels are safe or your organs are protected. Skipping tests is one of the most common reasons for late-stage complications.
Why is my doctor checking my cholesterol if I have a kidney transplant?
Some immunosuppressants, especially sirolimus and everolimus, raise cholesterol and triglycerides in 60-75% of patients. High cholesterol increases your risk of heart attack and stroke-already higher in transplant patients. Monitoring and treating it early helps you live longer, not just with a working kidney, but with a healthy heart too.
What is TTV monitoring, and should I ask for it?
TTV monitoring measures a harmless virus in your blood to estimate how suppressed your immune system is. Low TTV levels mean you’re at higher risk of rejection; high levels mean you’re more likely to get infections. It’s not standard everywhere yet, but it’s becoming more common in major transplant centers. If you’re on long-term immunosuppression, ask your doctor if TTV testing is available-it could help personalize your treatment and reduce complications.
Are there alternatives to frequent blood draws?
Right now, blood tests are still the only proven way to monitor drug levels and immune function. But research is moving fast. Point-of-care devices that use a finger-prick sample are in clinical trials and could be available by 2027. Non-invasive methods, like breath tests that detect drug metabolites, are being tested in labs. For now, blood tests are unavoidable-but they’re getting less painful and more accurate.




14 Comments
Lauren Scrima
Dec 13 2025So let me get this straight: I need to get poked 12 times in Year 1, pay $200 per test, and pray my lab doesn't use the cheap method that overcounts my tacrolimus by 20%?... And I'm supposed to be *grateful*? 😅
sharon soila
Dec 14 2025Every patient deserves the chance to live well. Monitoring is not a burden-it is a gift of time. With care, attention, and science, we turn fear into freedom. Keep going. You are stronger than you know. 🌱
nina nakamura
Dec 15 2025If you're not using LC-MS/MS you're doing harm. Immunoassays are garbage. Any center using them is either broke or negligent. No excuses. This isn't a debate. It's malpractice
Hamza Laassili
Dec 16 2025I mean come ONNNN-why do we even pay for all this? My cousin got a kidney and never did a single TTV test and he’s fine! America’s health system is just bleeding cash on overkill!!!
Constantine Vigderman
Dec 18 2025TTV monitoring?? I mean… that’s wild! 😲 Like… your body’s got its own little virus barometer?? I’m so hyped for this! 2025 can’t come soon enough!! 🚀💉
Cole Newman
Dec 19 2025You know what’s worse than the blood draws? The fact that your nurse doesn’t know what a C2 level is. I had to explain it to mine. Twice. Why are we trusting people who don’t understand the science?
Yatendra S
Dec 21 2025The virus… it watches us. TTV is not just a marker. It is the whisper of the immune system… silent… waiting… like the moon pulls the tide, so too does this tiny strand of DNA pull the balance of life and death. 🌌
Himmat Singh
Dec 22 2025It is regrettable that the prevailing paradigm in clinical practice continues to prioritize cost-efficiency over evidence-based precision. The utilitarian approach to therapeutic monitoring is fundamentally incompatible with the ethical imperative of patient safety.
Tommy Watson
Dec 23 2025I just got my 17th blood draw this month and my arm looks like a pin cushion. My insurance denied the LC-MS/MS test. I’m supposed to be grateful for a $50 test that gives me a 20% error rate?? This is a joke. A sick joke.
Richard Ayres
Dec 24 2025The integration of AI into monitoring protocols is one of the most promising developments in transplant care. Predicting rejection 14 days in advance isn't just impressive-it's transformative. We're moving from reactive to proactive medicine, and that’s a huge win.
Michael Gardner
Dec 24 2025I don't buy the TTV hype. If it were that reliable, it'd be standard everywhere. The fact that labs still can't agree on the method? That's not innovation. That's chaos.
Jade Hovet
Dec 26 2025I started logging my labs in a little notebook and now I feel like a boss. 📓✨ Also, I got my mom to do my lipids with me-she’s got diabetes too. We’re a team now. 💪❤️
nithin Kuntumadugu
Dec 27 2025TTV? LOL. Big Pharma’s new scam. They’re just trying to sell you more tests. The real reason you’re getting sick is because the transplant center is using cheap meds from China. I read it on a forum. It’s true.
John Fred
Dec 29 2025The AUC for MPA? That’s the golden ticket. If your doc doesn’t know what AUC means, find a new one. 🧪 This isn’t just science-it’s survival math. And yeah, I said math. 😎