Medication Excipient Safety Checker
This tool helps you determine if you might be sensitive to excipients in generic medications based on your medical history and medication type. It's especially important for patients with conditions affected by narrow therapeutic index drugs like thyroid medications or blood thinners.
This tool is based on the article content about excipients in medications. It's designed to help you identify potential risks but should not replace professional medical advice.
When you pick up a prescription, you might not think twice about whether it’s the brand-name version or the generic. After all, the FDA says they’re the same. But for some people, switching from a brand-name drug to a generic isn’t just a cost-saving move-it can trigger nausea, dizziness, worsening symptoms, or even allergic reactions. And the culprit isn’t the active ingredient. It’s the excipients.
What Are Excipients, and Why Do They Matter?
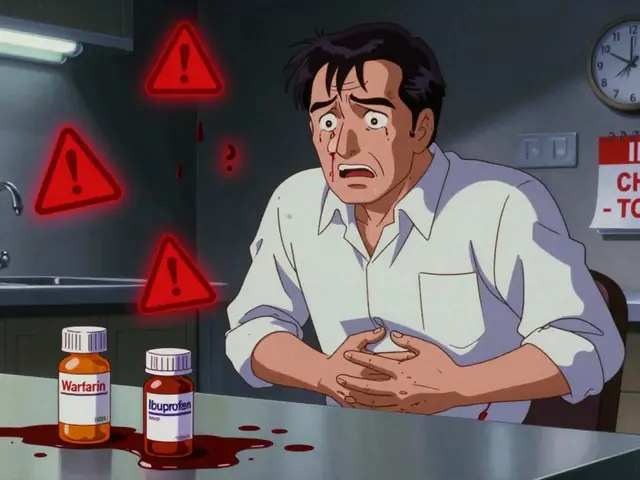
Excipients are the inactive ingredients in a medication. They don’t treat your condition. Instead, they help the drug stay stable, bind the pill together, make it easier to swallow, or give it color and flavor. Common excipients include lactose, cornstarch, dyes like FD&C Red No. 40, preservatives, and fillers like croscarmellose sodium or magnesium stearate. On paper, brand-name and generic drugs are supposed to be identical. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration. But when it comes to excipients? They can be completely different. That’s not a flaw in the system. It’s allowed by design. Generic manufacturers don’t have to copy the brand’s exact formula. They just need to prove their version delivers the same amount of active drug into the bloodstream within a narrow range-80% to 125% of the brand’s level. For most people, that’s plenty close enough. But for some, even small differences in how fast a pill dissolves or how the body absorbs the drug can make a big difference. That’s especially true for medications with a narrow therapeutic index (NTI), where the line between effective and toxic is thin.Narrow Therapeutic Index Drugs: When Small Changes Have Big Consequences
Drugs like warfarin (a blood thinner), levothyroxine (for thyroid conditions), and certain anti-seizure medications like phenytoin fall into this high-risk category. A 5% change in blood levels can mean the difference between controlling your condition and experiencing dangerous side effects. A 2022 study in JAMA Internal Medicine found that while 92% of generic drugs performed just as well as brand-name versions across 47 drug classes, the remaining 8% showed measurable differences in outcomes. These weren’t random. Most involved NTI drugs. Patients on levothyroxine, for example, have reported swings in thyroid levels after switching generics. One woman described her fatigue and weight gain returning after her pharmacy switched her to a cheaper version. Her doctor ordered a blood test-her TSH levels had jumped from 2.1 to 7.8. She switched back to the brand, and within weeks, she felt like herself again. Parkinson’s patients face similar challenges. Levodopa, the main treatment for Parkinson’s, is often sold as a combination with carbidopa. Generic versions may use different binders or coatings that change how quickly the drug dissolves in the gut. For someone whose body needs precise timing to avoid “off” periods-where movement becomes nearly impossible-those small delays can be devastating. Reddit users with Parkinson’s have shared stories of switching to a generic version of Sinemet or Rytary and suddenly experiencing tremors, confusion, or nausea. Some reported dramatic improvement after going back to the brand. One user wrote: “I didn’t realize how much I’d been struggling until I switched back. It was like someone turned the lights on.”Excipient Allergies and Intolerances: More Common Than You Think
It’s not just about absorption. Some people have real allergies or intolerances to excipients. Lactose is one of the most common offenders. It’s used as a filler in about 20% of oral medications. For someone with lactose intolerance, even a small amount can cause bloating, cramps, or diarrhea. One patient with hypothyroidism switched to a generic levothyroxine that contained lactose. Within days, she had severe stomach pain and couldn’t keep food down. Her doctor assumed it was a stomach bug. It wasn’t. It was the pill. Another case involved a patient with a known allergy to croscarmellose sodium-a common disintegrant in pills. After switching from a brand-name furosemide (a water pill) to a generic version, she developed hives and swelling. The brand version didn’t contain it. The generic did. Once she switched back, the reaction stopped. Dyes are another hidden trigger. Red, yellow, and blue food dyes are often used to distinguish pills by dose or manufacturer. People with dye sensitivities have reported rashes, headaches, and even asthma attacks after switching to a generic version with a different color. The American College of Allergy, Asthma, and Immunology estimates that 15 to 20 million Americans have significant excipient intolerances. Yet, most doctors and even pharmacists don’t routinely ask about them.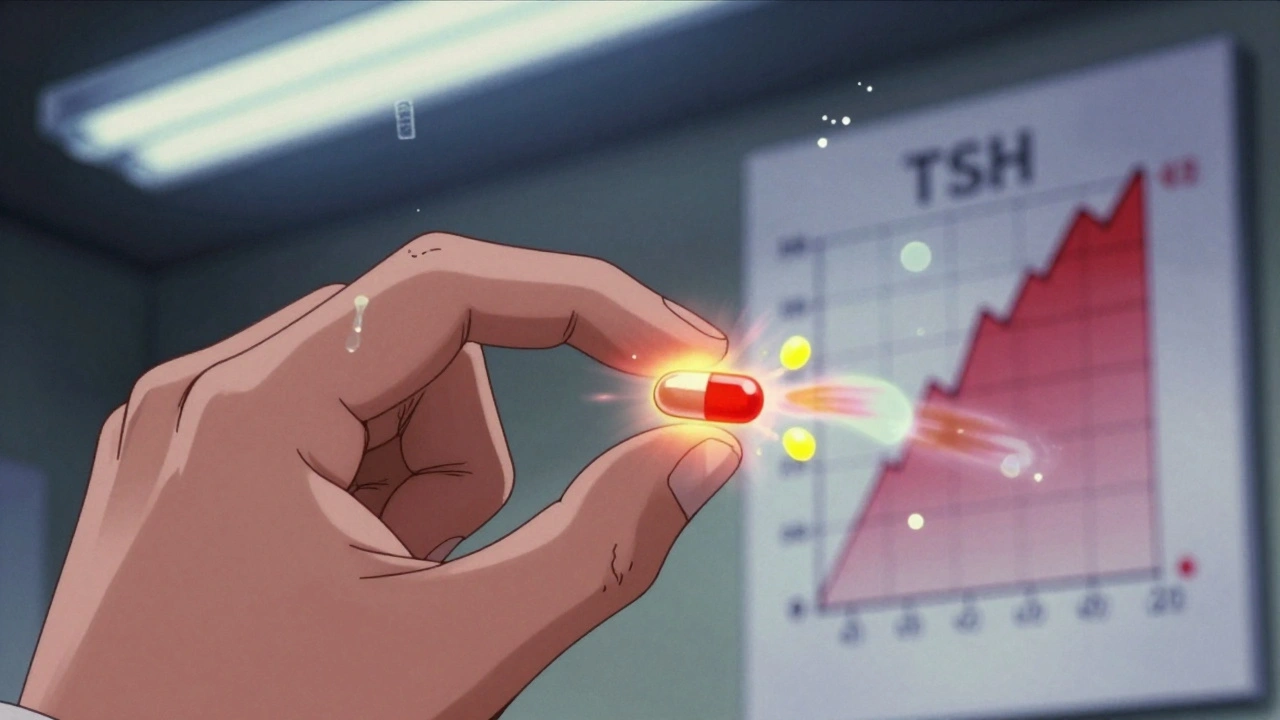
Why Don’t We Know What’s in Our Pills?
Here’s the frustrating part: you won’t find a full list of excipients on the pill bottle. The label only says the drug name and dosage. To see what’s inside, you need to dig deeper. You can check the package insert that comes with the medication. It’s usually a small booklet with fine print. Or ask your pharmacist for the “Product Information Sheet” from the manufacturer. Some pharmacies have this on file. Others don’t. And even then, manufacturers can change excipients without telling you. That’s why keeping a medication diary matters. Write down: the name of the drug (brand or generic), the manufacturer, the date you started, and any new symptoms. If you feel worse after a switch, you’ll have proof to show your doctor.What Can You Do If You Suspect an Excipient Problem?
First, don’t assume your reaction is “all in your head.” If you’ve had no issues with a brand-name drug and suddenly feel worse after switching to a generic, it’s worth investigating. Talk to your pharmacist. Ask: “Is this the same manufacturer as before?” and “What excipients are in this version?” If they don’t know, ask them to look up the product information. Pharmacists are trained to know this stuff. Ask your doctor to write “dispense as written” or “do not substitute” on your prescription. That legally prevents the pharmacy from swapping your brand for a generic without your consent. If you’re on a high-risk medication like warfarin or levothyroxine, ask if you can stick with the same generic manufacturer every time. Some companies make more consistent formulations than others. Sticking with one brand of generic can reduce the chance of unexpected changes. And if you’ve had a reaction, report it. The FDA’s MedWatch program lets patients report adverse events. Your report helps regulators track patterns and possibly update guidelines.
Is There Hope for Better Transparency?
Yes. The FDA is working on a public database that will list excipients for both brand and generic drugs. It’s still in development, but when it launches, you’ll be able to look up a drug and see exactly what’s in it-no digging required. Some generic manufacturers are already responding to demand. In early 2024, the Generic Pharmaceutical Association announced that members will start standardizing excipients in NTI drugs to reduce switching problems. One company even began marketing its generic thyroid medication as “lactose-free and dye-free” to appeal to sensitive patients. By 2030, experts predict “clean excipient profiles” will become a selling point-just like gluten-free or organic labels on food.Bottom Line: Generics Are Safe-For Most People
For the vast majority of patients, generics are just as safe and effective as brand-name drugs. They’ve saved the U.S. healthcare system over $370 billion a year. That’s money that keeps treatments affordable for millions. But for the 5 to 7% of patients who experience issues-often due to excipients-those savings come at a cost. Your body isn’t just reacting to the active ingredient. It’s reacting to the whole package. If you’ve ever felt worse after switching to a generic, you’re not imagining it. You’re not being difficult. You’re part of a group that’s been overlooked. Ask questions. Keep track. Speak up. Your health isn’t just about what the drug treats-it’s about what the drug is made of.Are generic medications always as effective as brand-name drugs?
For most people, yes. The FDA requires generics to meet strict bioequivalence standards, meaning they deliver the same active ingredient at the same rate and amount as the brand. Studies show 92% of generics perform just as well across 47 drug classes. But for medications with a narrow therapeutic index-like warfarin, levothyroxine, or anti-seizure drugs-small differences in absorption can matter. In those cases, some patients experience reduced effectiveness or side effects after switching.
Can excipients in generic drugs cause allergic reactions?
Yes. While rare, excipients like lactose, dyes (e.g., FD&C Red No. 40), and preservatives can trigger allergic or intolerance reactions in sensitive individuals. Cases have been documented where patients developed hives, gastrointestinal distress, or worsening symptoms after switching to a generic that contained an excipient they were sensitive to-even though the brand version didn’t have it.
How do I find out what excipients are in my medication?
Check the package insert that comes with your prescription. If you don’t have it, ask your pharmacist for the manufacturer’s Product Information Sheet. You can also search the FDA’s online database for drug labeling. Keep in mind: manufacturers can change excipients without notice, so it’s wise to verify each time you refill.
Can my doctor prevent my pharmacy from switching my brand to a generic?
Yes. Your doctor can write “dispense as written” or “do not substitute” on your prescription. This legally requires the pharmacy to fill it with the exact drug specified, even if a generic is available. This is especially important if you’ve had a bad reaction to a generic version in the past.
Should I avoid generic medications if I have a food intolerance?
If you have a known intolerance-like lactose intolerance, celiac disease, or dye sensitivity-it’s wise to check excipients before switching to a generic. Many medications contain lactose, cornstarch, or artificial colors. Talk to your pharmacist about your specific intolerance and ask for a version that avoids those ingredients. You don’t have to avoid generics entirely, but you need to be selective.






16 Comments
Dematteo Lasonya
Dec 2 2025I switched to a generic levothyroxine last year and my TSH went from 3.2 to 9.1 in six weeks. My doctor thought I was noncompliant. I had to fight for months to get my original brand back. Turns out the generic had lactose. I’m lactose intolerant. No one asked. No one cared.
Gillian Watson
Dec 2 2025Here in the UK, we get generics all the time and never think twice. But I’ve got a mate with epilepsy who swears his seizures spiked after a switch. He’s now on a private prescription just to keep the same maker. Weird how something so small can wreck your life.
zac grant
Dec 3 2025From a pharmacokinetics standpoint, bioequivalence (80–125% AUC) is statistically sound for population-level outcomes-but it’s a terrible metric for individualized medicine. NTI drugs like warfarin or phenytoin have coefficient of variation thresholds below 10%. When excipient variability alters dissolution kinetics, you’re not just ‘close enough’-you’re clinically unstable. The FDA’s one-size-fits-all framework ignores interindividual pharmacodynamic heterogeneity. This isn’t a flaw in generics-it’s a flaw in regulatory philosophy.
Carolyn Ford
Dec 4 2025Oh here we go. Another ‘I’m special and my body is magic’ story. People who can’t handle a little lactose in a pill should just stop taking medicine altogether. It’s not the drug’s fault you’re a hypochondriac.
Dematteo Lasonya
Dec 5 2025Carolyn, I didn’t say I was special. I said I was sick. And my doctor confirmed it with labs. You think I wanted to spend three months in pain because some pharmacist thought ‘same active ingredient’ meant ‘same everything’?
Jessica Baydowicz
Dec 5 2025OMG YES. I had hives after switching to a generic furosemide. Turns out the generic had FD&C Yellow No. 5. The brand? None. I cried. My skin looked like a strawberry. My pharmacist didn’t even know the difference. We need labels like food. Like, ‘Contains: Dyes, Lactose, Corn’-no hiding behind ‘inactive ingredients’.
Ashley Elliott
Dec 7 2025Thank you for writing this. I’ve been a nurse for 18 years and I’ve seen this over and over. Patients say ‘I feel weird’ and we blame stress or aging. We don’t ask about pills. We should. It’s not just about cost-it’s about dignity.
Yasmine Hajar
Dec 8 2025My mom’s on levothyroxine. She went from feeling fine to exhausted, gaining 15 lbs, and crying for no reason. We found out the pharmacy switched her to a generic with cornstarch and magnesium stearate. She went back to the brand-within 10 days, she was herself again. This isn’t ‘in your head.’ It’s science. And it’s being ignored.
Karl Barrett
Dec 9 2025There’s a deeper philosophical issue here: if we treat the body as a black box that only responds to active ingredients, we’re reducing medicine to chemistry. But humans are complex systems-biochemistry, microbiome, immune reactivity, even psychological expectation all interact. The excipient isn’t ‘inactive’-it’s context. And context matters.
Rudy Van den Boogaert
Dec 11 2025I’m on a generic for my blood pressure med. No issues. But I know someone whose kid had a rash after switching. They tracked it to a dye. Took forever to figure out. Just something to keep in mind. Not everyone’s the same.
Bill Wolfe
Dec 13 2025Wow. Another cry for special treatment. Did you know that 92% of people don’t have issues? You’re not a victim-you’re an outlier who doesn’t want to pay $5 instead of $20. Maybe you should just stop being so fragile and learn to adapt. The world doesn’t revolve around your digestive system.
Alex Piddington
Dec 15 2025For those asking how to check excipients: I use DailyMed (dailymed.nlm.nih.gov). You can search by drug name and download the SPL (Structured Product Labeling) file. It lists every excipient, even the trace ones. It’s tedious, but it’s free and official. Also, pharmacists are legally required to provide this upon request.
Pavan Kankala
Dec 16 2025Big Pharma doesn’t want you to know this. Generics are cheaper because they’re made in unregulated factories overseas. The ‘active ingredient’ might be real-but the excipients? Who knows what’s really in there. Glyphosate. Heavy metals. Microplastics. They don’t test for it. They just print ‘FDA approved’ and call it a day. You’re being experimented on.
Jake Deeds
Dec 17 2025It’s tragic. We’ve turned healthcare into a spreadsheet. ‘Can we make this pill cheaper?’ No one asks ‘Will this pill kill someone quietly?’ I’ve lost three friends to this. One had a seizure because the generic dissolved too slow. They called it ‘sudden cardiac arrest.’ It wasn’t. It was a pill.
Jordan Wall
Dec 19 2025Bro. You’re telling me that some guy in China who makes pills for $0.02 a tablet can’t get the same filler as the US brand? Lol. That’s why I pay extra for brand. You think the FDA checks every batch? Nah. They sample 0.001%. You’re a lab rat. Enjoy your $3 prescription.
michael booth
Dec 20 2025Thank you for sharing this vital information. As a healthcare professional, I urge everyone to document changes in medication, report adverse events to MedWatch, and advocate for transparency. Your voice matters. Small steps lead to big change.